Recovery of Metals
Our work on metals currently focusses on copper and zinc. Copper and zinc are present in waste streams from a variety of industries. Before wastewaters can be discharged into the water environment, they must be treated to remove metals to avoid negative effects on the environment. There are strict discharge limits for both copper and zinc.
 We are researching the use of Bioelectrochemical systems (BES) to remove metals. We are optimising the systems to minimise the additional energy input to recover metals so that they can be recycled and reused within the economy.
We are researching the use of Bioelectrochemical systems (BES) to remove metals. We are optimising the systems to minimise the additional energy input to recover metals so that they can be recycled and reused within the economy.
We are using the BES to recover pure copper at the cathode from distillery 'spent lees' (the wastewater from the bottom of the copper stills used to distill alcohol for whisky making). The bacteria in the anode chamber provide a current (a flow of electrons) by consuming organic matter in wastewater. In the cathode chamber, the copper ions in solution pick up the electrons and form solid copper which plates onto the electrode.
In addition to purely electrochemical recovery of metals we are utilizing biocathodes for development of more sophisticated metal recovery processes.
Metalreducing bacteria are well known for their capacity to interact with electrodes. Typically organisms such as Geobacter spp. or Shewanella spp. are applied in the anodes of a bioelectrochemical system (BES) where they oxidize organic electron donors such as acetate or lactate and donate electrons to an anode generating electrical current with the donated electrons ultimately used to reduce oxygen at the cathode, usually catalyzed by a platinum catalyst. However these organisms can also accept electrons from electrodes and thus in the cathode of a BES they may be used to extract electrons from a wastewater-fed anode.
Novel materials
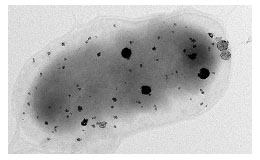 More than simply reducing metals some metal-reducing bacteria produce highly organized metallic nanostructures with specific properties that make them suitable for use in a wide range of applications. Such “functional bionanominerals” include nanocatalaysts, quantum dots and nanomagnets with highly tunable properties. Conventional processes to produce functional nanomaterials use high value feedstocks and require highly controlled conditions, often in extreme processing conditions (e.g. pressures and temperatures). Producing such materials under ambient conditions from a complex feedstock such as wastewater is an attractive proposition since it could be used to manufacture a high value product from a low value resource in an environmentally sustainable process.
More than simply reducing metals some metal-reducing bacteria produce highly organized metallic nanostructures with specific properties that make them suitable for use in a wide range of applications. Such “functional bionanominerals” include nanocatalaysts, quantum dots and nanomagnets with highly tunable properties. Conventional processes to produce functional nanomaterials use high value feedstocks and require highly controlled conditions, often in extreme processing conditions (e.g. pressures and temperatures). Producing such materials under ambient conditions from a complex feedstock such as wastewater is an attractive proposition since it could be used to manufacture a high value product from a low value resource in an environmentally sustainable process.