Microbial Electrochemical Technology
Microbial electrochemical technology is a rapidly growing environmental technology, bringing together the disciplines of microbiology, electrochemistry, materials science and engineering. It can be applied to a range of environmental challenges – water treatment, recovery of resources from waste water, synthesis of valuable and novel chemicals and fuels and clean energy generation.
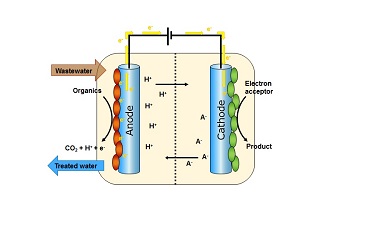 Microbial electrochemical technology uses Bioelectrochemical systems (BES). These are engineered systems that use microorganisms to convert the chemical energy stored in biodegradable organic matter to direct electric current and chemicals. The BES consists of two chambers separated by a membrane. Each chamber has an electrode and these are coupled together by conductive wire. The so-called anode chamber contains the microorganisms and wastewater which contains biodegradable matter, such as the organic matter in dirty domestic water. As microorganisms oxidise biodegradable substances they release electrons. Normally, the electrons would naturally flow towards oxygen or some other electron acceptor present in the water, but it the BES, the electrons are captured by an electrode (the anode) and they flow to the electrode (the cathode) in the other chamber. Depending on what substances are present in the cathode chamber, the electrons can be used in a variety of ways.
Microbial electrochemical technology uses Bioelectrochemical systems (BES). These are engineered systems that use microorganisms to convert the chemical energy stored in biodegradable organic matter to direct electric current and chemicals. The BES consists of two chambers separated by a membrane. Each chamber has an electrode and these are coupled together by conductive wire. The so-called anode chamber contains the microorganisms and wastewater which contains biodegradable matter, such as the organic matter in dirty domestic water. As microorganisms oxidise biodegradable substances they release electrons. Normally, the electrons would naturally flow towards oxygen or some other electron acceptor present in the water, but it the BES, the electrons are captured by an electrode (the anode) and they flow to the electrode (the cathode) in the other chamber. Depending on what substances are present in the cathode chamber, the electrons can be used in a variety of ways.
The current can be captured directly for electricity generation (microbial fuel cells, MFCs) or used to produce hydrogen gas. The electrons can also be used in the cathode chamber to reduce dissolved metals in waste water, resulting in the pure metal being deposited onto the cathode where it can then be recovered for reuse. The electrons can also be used to reduce carbon dioxide to synthesize useful organic compounds such as methanol, which is a useful chemical feedstock and fuel.
Click for more information on Recovery of Metals and Making Products from Carbon Dioxide